.jpg)


.jpg)




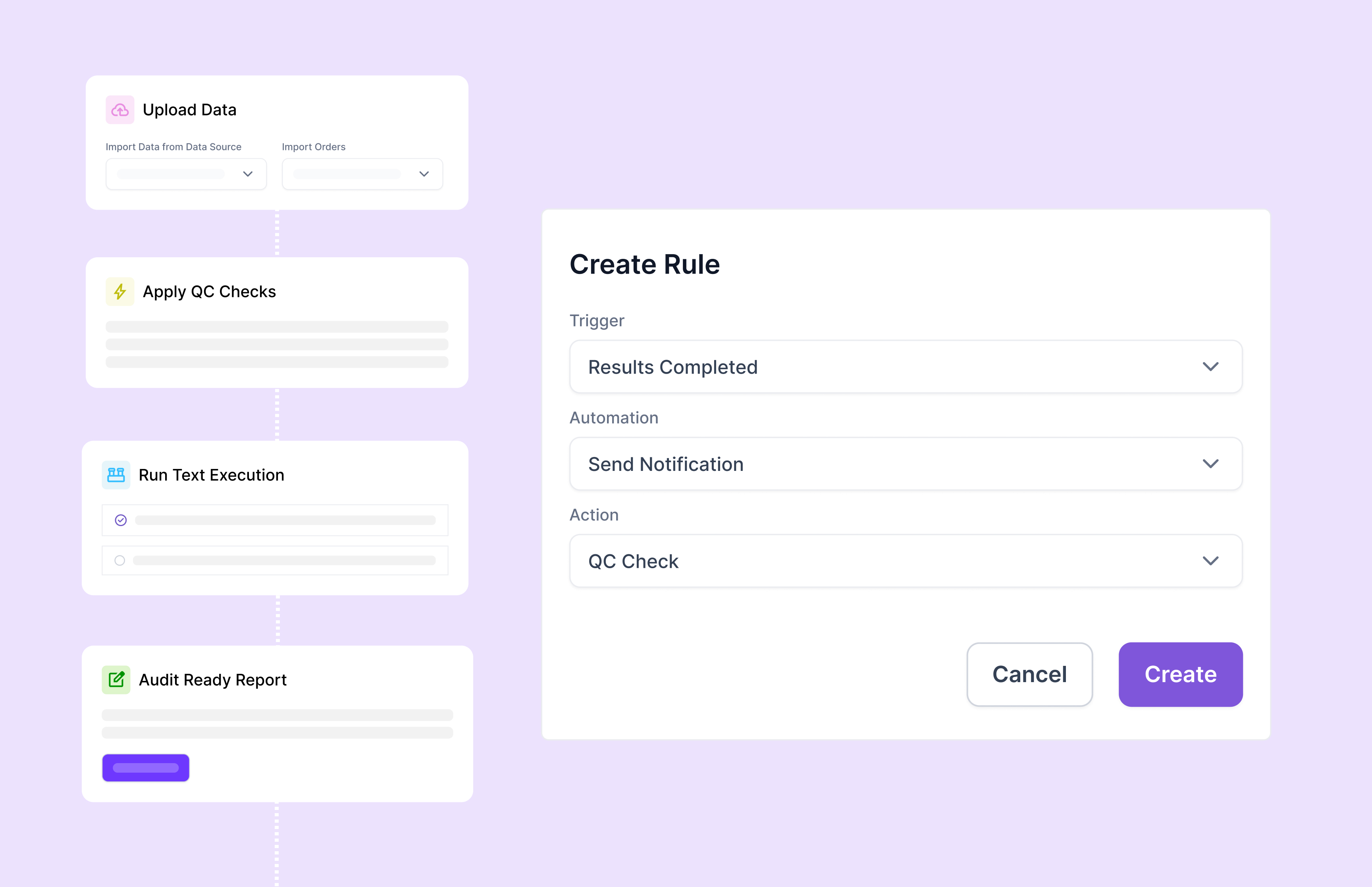
GxP compliance refers to regulatory standards, including Good Laboratory Practices (GLP), Good Manufacturing Practices (GMP), and Good Clinical Practices (GCP). These standards ensure product quality, patient safety, reliable data generation, and regulatory adherence, crucial for regulated life sciences sectors such as pharmaceuticals, biotech, and clinical diagnostics.
Scispot’s GxP compliance software provides comprehensive audit trails, 21 CFR Part 11-compliant electronic signatures, detailed version control, automated workflows, and role-specific access permissions. It helps organizations fully comply with regulatory standards including FDA's 21 CFR Part 11, EU Annex 11, ISO certification, and SOC 2, substantially reducing regulatory compliance risks.
Yes, Scispot’s sample management software is designed to integrate seamlessly with other laboratory systems, including Electronic Lab Notebooks (ELNs), Laboratory Information Management Systems (LIMS), and data analysis platforms. This integration ensures smooth data flow and collaboration across all aspects of laboratory operations.
Yes, Scispot’s GxP compliance platform is ideal for biotech startups, CROs, diagnostics labs, and enterprise-scale operations. The user-friendly, no-code configuration system simplifies the management of compliance workflows, enabling labs to efficiently maintain regulatory compliance without extensive IT support or resources.
Scispot’s GxP compliance software ensures robust data security through end-to-end encryption, secure cloud storage, real-time system monitoring, and strict role-based access permissions. These security measures align with data security and traceability requirements stipulated by GxP regulations, HIPAA, and SOC 2.
Yes, Scispot fully supports electronic signatures compliant with 21 CFR Part 11 regulations. Users can digitally approve, securely sign, and reliably track documentation with detailed timestamped records, maintaining full auditability and ensuring a comprehensive chain of custody throughout regulatory processes.
Scispot streamlines audit preparation by automatically generating tamper-proof audit trails, customizable compliance reports, and centralized document controls. It also integrates quality control checks and deviation reporting, significantly enhancing audit readiness and ensuring swift retrieval of essential documentation during regulatory inspections.