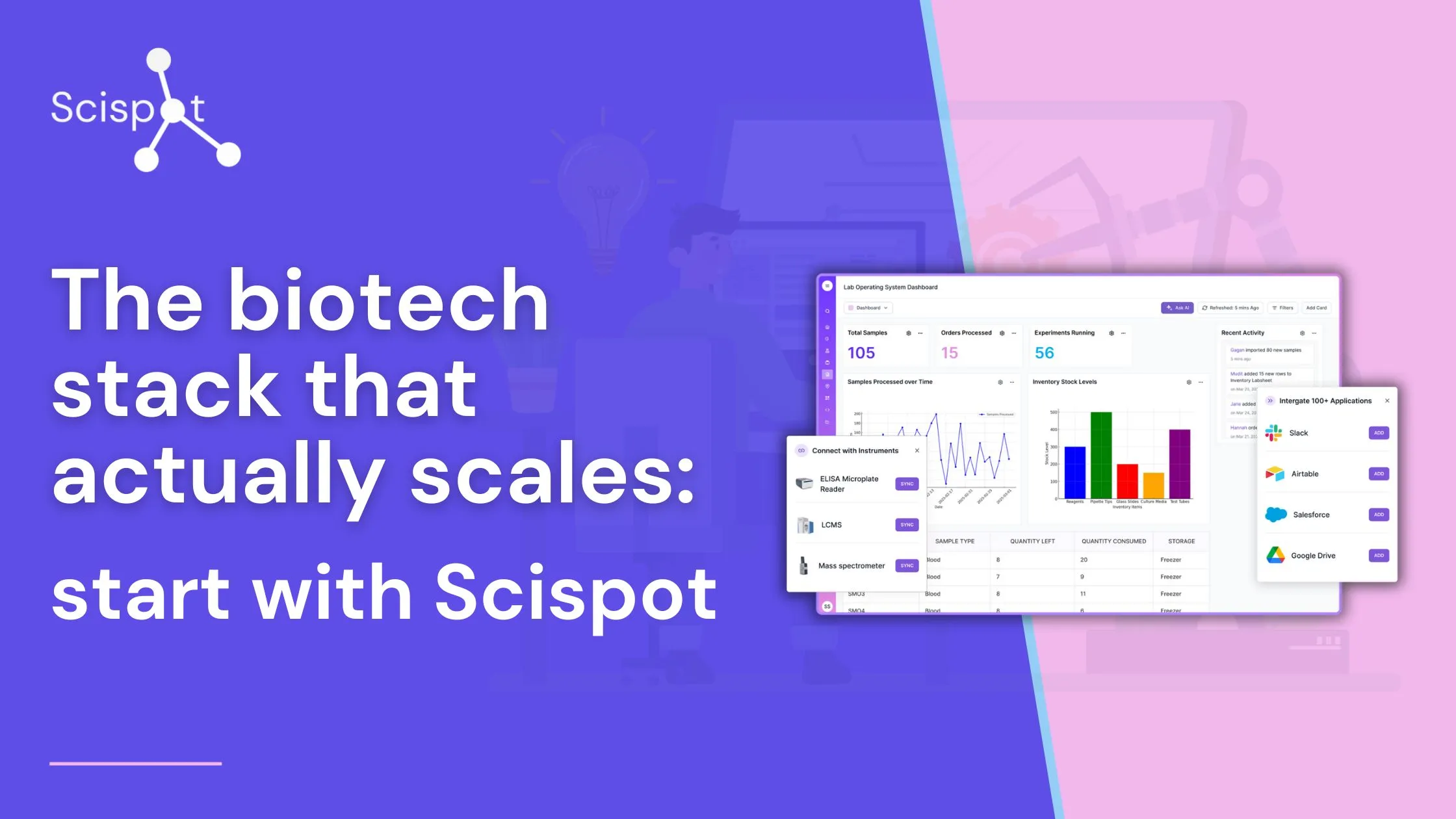
When laboratories decide to adopt a Laboratory Information Management System (LIMS), the process often feels daunting. However, LIMS implementation doesn’t have to be overwhelming if approached with a well-thought-out strategy. In this guide, we’ll break down everything you need to know about implementing LIMS, addressing potential hurdles, and offering actionable insights to make your journey smoother.
What is LIMS Implementation?
LIMS implementation refers to integrating a Laboratory Information Management System into your lab’s daily operations. This involves configuring the system to meet specific workflows by customizing its features, such as data fields, user roles, and permissions, to align with lab operations. It also includes integrating the LIMS with existing tools like laboratory instruments, ELNs, and ERP systems, ensuring seamless data flow. Additionally, the process requires training staff, migrating legacy data while maintaining its integrity, and ensuring compliance with industry standards.
Every laboratory is unique, which means no two implementations are the same. For example, a diagnostics lab may prioritize compliance with strict regulatory standards and integration with instruments like PCR machines. In contrast, a research lab might focus on flexibility for exploratory workflows and compatibility with data analysis tools like Jupyter Notebooks. Whether you’re in diagnostics, pharmaceuticals, or academic research, the success of your LIMS project plan hinges on customization and a clear roadmap.
Why is LIMS Implementation Essential?
A well-executed implementation of LIMS transforms how laboratories operate. It centralizes data, automates workflows, and ensures better compliance and traceability. Here’s why it matters:
- Enhanced Efficiency: Automating routine tasks saves time and minimizes errors.
- Centralized Data: All lab information is easily accessible, reducing time spent searching for records.
- Regulatory Compliance: A robust LIMS aligns with FDA, GxP, HIPAA, or ISO standards.
- Scalability: A sound system grows with your lab, adapting to increased volumes and complexities.

Metadata and barcoding improve sample tracking. Scispot's centralized sample management makes managing patient biopsies, environmental swabs, and reagent inventories easy.
Furthermore, laboratories with an effective LIMS can reduce operational costs, improve data accuracy, and accelerate decision-making. In a competitive environment, a well-implemented LIMS gives labs the edge needed to stay ahead.
However, challenges can arise without proper preparation, so let’s explore those next.
Top LIMS Implementation Challenges
- Data Migration Issues: Moving legacy data to the new LIMS can be complex if the data is unstructured, incomplete, or stored in incompatible formats. Labs often underestimate the time required for data cleanup and standardization.
- Resistance to Change: Staff may hesitate to adapt to new workflows, especially if they are accustomed to manual processes or older systems.
- Underestimating Time and Cost: Implementation timelines can spiral without a detailed LIMS project plan, leading to missed deadlines and budget overruns.
- Vendor Selection: Choosing the wrong vendor can lead to misaligned features, inadequate support, and costly revisions. Labs must ensure their chosen LIMS can handle their specific workflows and integrate with their existing infrastructure.
- Integration Complexity: A LIMS must seamlessly integrate with existing instruments and software, such as ELNs, ERPs, or specific lab tools. Failure to achieve this can result in disconnected systems and inefficiencies.
- Training and User Adoption: Even the best LIMS can fail without proper user training. Employees must understand how to use the system and how it benefits their work.
Partnering with Scispot, which specializes in rapid deployment and tailored solutions, can help you overcome these challenges efficiently. For instance, a California cannabis testing lab achieved a 50% increase CoA turnaround and a 80% reduction in data entry errors by implementing Scispot alt-LIMS.
.png)
How to Implement LIMS Successfully
To ensure smooth LIMS implementation, follow these key steps:
1. Define Your Goals
Start by clearly understanding why you need a LIMS. Are you looking to improve data accuracy, simplify compliance, or increase throughput? Knowing your objectives helps shape the LIMS project plan.
2. Assemble a Cross-Functional Team
Involve stakeholders from various departments—IT, QA/QC, lab managers, and end users. Their input ensures the system addresses everyone’s needs. A well-rounded team fosters better decision-making and smoother adoption.
3. Map Your Workflows
Document existing workflows in detail. Identify gaps, redundancies, and areas where automation could add value. For instance, a lab might find that manual data entry leads to frequent errors and delays. Implementing LIMS to automate data capture from instruments and standardize reporting can significantly reduce these inefficiencies, ensuring accuracy and faster turnaround times. This map will guide system configuration and ensure the LIMS aligns with your specific operational needs.
4. Choose the Right Vendor
The vendor’s experience in your industry and ability to deliver fast, customized solutions are crucial. Ask for demos, request case studies, and verify the vendor’s expertise. Scispot’s flexible platform, for example, integrates seamlessly with instruments and databases, providing unmatched scalability.

5. Develop a Comprehensive LIMS Project Plan
Include timelines, milestones, resources, and contingencies. A solid plan keeps the team aligned and accountable. For example, allocate specific phases for data migration, testing, and staff training to avoid bottlenecks.
6. Train Your Team
Comprehensive training reduces resistance and improves user adoption. Provide hands-on workshops and easily accessible resources to make the transition smooth. Ensure that training is tailored to each team’s role within the system.
7. Perform Validation and Testing
Ensure the LIMS meets all functional and regulatory requirements. Testing should cover data integrity, workflow alignment, system security, and instrument integration. Simulate real-world scenarios to uncover potential issues before going live.
8. Go Live in Phases
Rolling out the system in phases minimizes disruptions. Start with one department or workflow, gather feedback, and refine before full deployment. A phased approach also helps build confidence among staff as they see the system’s benefits in action.
Why is Scispot the fastest LIMS to implement?
At Scispot, we understand laboratories don’t have the luxury of lengthy implementations. That’s why we’ve designed our solutions to deliver the fastest implementation of LIMS without compromising on quality.
Our approach includes:
- Pre-Built Templates: Scispot offers pre-configured workflows tailored to specific industries, such as molecular diagnostics, biobanking, and quality control. These templates accelerate implementation by providing a solid foundation for sample tracking, assay management, and compliance documentation. For example, a molecular diagnostics lab that utilized our templates experienced a 30% reduction in setup time and immediate improvements in sample throughput by automating accessioning processes and generating standard operating procedures with minimal customization.
- AI-Powered Workflows: Automate routine tasks like sample accessioning, data entry, and compliance reporting. This not only improves efficiency but also ensures consistent quality.
- Seamless Integrations: Our platform integrates with popular lab instruments and software, such as Netsuite, Zapier, Monday.com, and more than 7,000 apps. This ensures that your lab’s ecosystem remains connected and efficient.
- Flexible Deployment: Whether you need cloud-based or on-premise solutions, we adapt to your needs, offering scalability for labs of all sizes.
- Expert Support: Our team guides you every step of the way, from planning to post-implementation support. This hands-on approach minimizes downtime and ensures a smoother transition.
With Scispot, you can have a fully operational LIMS in weeks rather than months, enabling your lab to focus on what matters most: science.
By investing in the right LIMS solution and following a structured approach, your laboratory can unlock new levels of efficiency, compliance, and scalability. Ready to start your LIMS journey? Contact Scispot to schedule a demo or access a free guide on how our solutions can transform your lab operations quickly and efficiently.