Quality assurance failures in biotech can be costly, both financially and reputationally. For example, in 2019, Novartis received an FDA warning letter due to data integrity violations in product testing, which led to regulatory scrutiny and delays in drug approvals. due to inconsistent product testing and data integrity issues, resulting in halted production and millions in lost revenue. Such failures highlight the need for robust biotech quality assurance (QA biotech) systems that ensure compliance, product safety, and operational efficiency.
Without stringent QA processes, biotech firms risk product recalls, legal consequences, and regulatory scrutiny. The industry has seen a shift toward automation and AI-driven quality management systems that proactively detect anomalies, prevent non-compliance, and streamline workflows. Companies that embrace modern quality assurance biotech solutions can enhance their reputation, reduce costs, and accelerate product development. According to a report by McKinsey, generative AI in the pharmaceutical industry can improve quality assurance processes, reduce human error, and enhance compliance efficiency.
Maintaining high-quality standards in the biotechnology sector is essential to ensuring product safety, regulatory compliance, and operational efficiency. In an industry where even minor deviations can lead to costly recalls and regulatory scrutiny, robust quality assurance processes are a necessity rather than an option. Biotech quality assurance (QA biotech) encompasses systematic processes to ensure products meet predefined standards, ensuring safety, efficacy, and regulatory compliance. Without robust QA systems, biotech firms risk product recalls, legal consequences, and damage to their reputation.
Modern QA biotech practices involve a combination of automation, real-time monitoring, and structured documentation. For example, Moderna, a leading biotech firm, has successfully implemented AI-driven quality assurance systems to streamline vaccine production. By integrating automated monitoring and digital compliance tracking, they improved batch consistency, reduced errors, and ensured faster regulatory approvals. This demonstrates how leveraging modern QA biotech practices can enhance efficiency and reliability in biotech manufacturing. Companies that implement effective quality assurance frameworks not only comply with global regulations but also enhance operational efficiency and build trust with consumers and regulatory bodies.
Historically, biotech companies relied on paper-based quality management systems, which were prone to human error and inefficiencies. Today, digital transformation is reshaping quality assurance biotech with AI-driven insights, cloud-based data management, and automated compliance tracking. This shift enables biotech firms to identify quality issues proactively, streamline workflows, and scale operations efficiently.
The Importance of Quality Assurance in Biotechnology
Quality assurance in biotech is not merely a regulatory obligation but a foundational element that influences product development, manufacturing, and distribution. Ensuring high quality in biotech products is critical, as even minor inconsistencies can lead to failed clinical trials, rejected regulatory approvals, or compromised patient safety. A molecular diagnostic company faced challenges with fragmented data and manual processes, leading to inefficiencies and potential for errors. By implementing Scispot's alt-SDMS, they centralized their data management, automating data integration and enhancing data quality. This transformation resulted in improved data accuracy and streamlined operations, significantly reducing the potential for human error.

Regulatory compliance is at the core of biotech quality assurance. Organizations such as the FDA (Food and Drug Administration), EMA (European Medicines Agency), and WHO (World Health Organization) impose stringent quality control measures. Companies must comply with Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP), and ISO 13485 for medical devices to ensure product safety and reliability. Non-compliance can lead to hefty fines, production shutdowns, and legal liabilities.
Product safety and risk management are integral to biotech operations. Strict quality control ensures that contaminants, incorrect formulations, or unforeseen reactions are identified early. Implementing robust QA biotech measures enables companies to reduce risks of defective batches, product recalls, and regulatory penalties.
Data integrity and traceability are essential aspects of quality assurance biotech. Maintaining clear, accessible, and audit-ready records ensures compliance with regulatory bodies and facilitates faster approvals. Organizations using digital systems for data management significantly reduce errors associated with manual documentation.

Market reputation and consumer trust rely heavily on stringent QA biotech protocols. Companies that consistently deliver high-quality, compliant products gain credibility, leading to increased partnerships and customer confidence. A biotech firm’s reputation hinges on the reliability of its products, making QA a key differentiator in competitive markets.
Operational efficiency and cost savings are direct benefits of structured QA biotech processes. Automated workflows, standardized procedures, and digital tracking systems help reduce operational waste and errors. When deviations are identified early, companies avoid expensive corrective actions, rework, and compliance issues.
Investing in quality assurance biotech is not just a regulatory requirement but a strategic move that strengthens a company's ability to innovate, scale, and meet industry demands. With automation, AI-driven analytics, and robust compliance management, biotech firms can accelerate product development while ensuring high quality and safety.
Challenges in Implementing QA and QC in Biotech
Fragmented data management is a major challenge. Many companies store data in spreadsheets or across multiple systems, making compliance and traceability difficult. Data silos prevent seamless collaboration between teams and increase the risk of non-compliance. A centralized digital system is essential for maintaining data integrity and audit readiness. A preclinical contract research organization (CRO) faced challenges with data fragmentation and inconsistencies, impacting the quality of their research. By implementing Scispot's solutions, they centralized their data management, resulting in a threefold improvement in data quality. This enhancement facilitated more accurate analyses and supported compliance with industry standards.
Lack of standardization is another common issue. Variability in processes across different teams, departments, and locations can result in inconsistencies in quality control. Standard operating procedures (SOPs) and centralized digital documentation are essential to maintaining uniformity. Without these measures, deviations in protocols can lead to regulatory risks and inconsistencies in product quality.
Compliance complexity poses a significant burden on biotech firms. Navigating a web of regulatory requirements such as FDA 21 CFR Part 11, ISO 13485, HIPAA, and GxP compliance demands extensive documentation, validation, and continuous monitoring. Without an automated system to track compliance requirements, biotech companies risk audits, penalties, and production delays. The FDA issued over 1,000 warning letters in 2022 related to non-compliance demonstrating the high stakes of regulatory adherence.
Manual processes and human error further hinder quality assurance efforts. Many biotech companies still rely on paper-based records, increasing the risk of data entry errors, missing documentation, and inefficiencies. Automated digital solutions reduce administrative overhead and improve accuracy in quality tracking. A study by McKinsey found that automating quality management can reduce human error-related deviations by up to 50%.
Scalability challenges emerge as biotech companies grow. Small biotech firms often rely on ad-hoc QA/QC processes that become unsustainable as they scale. Without a scalable digital infrastructure, expanding operations leads to inconsistent quality control, compliance bottlenecks, and increased operational costs. Companies that adopt scalable QA solutions early report smoother expansion and fewer operational bottlenecks.
Integration with laboratory instruments and data systems is another roadblock. Many biotech firms use a mix of legacy and modern lab equipment that does not communicate seamlessly. A lack of integration between LIMS, ELN, and QC systems creates inefficiencies, delays, and increased manual work.
Despite its importance, many biotech firms struggle with effective QC biotech processes.
To overcome these challenges, biotech firms must adopt AI-driven, cloud-based, and automated quality management solutions that integrate compliance tracking, real-time monitoring, and workflow automation.
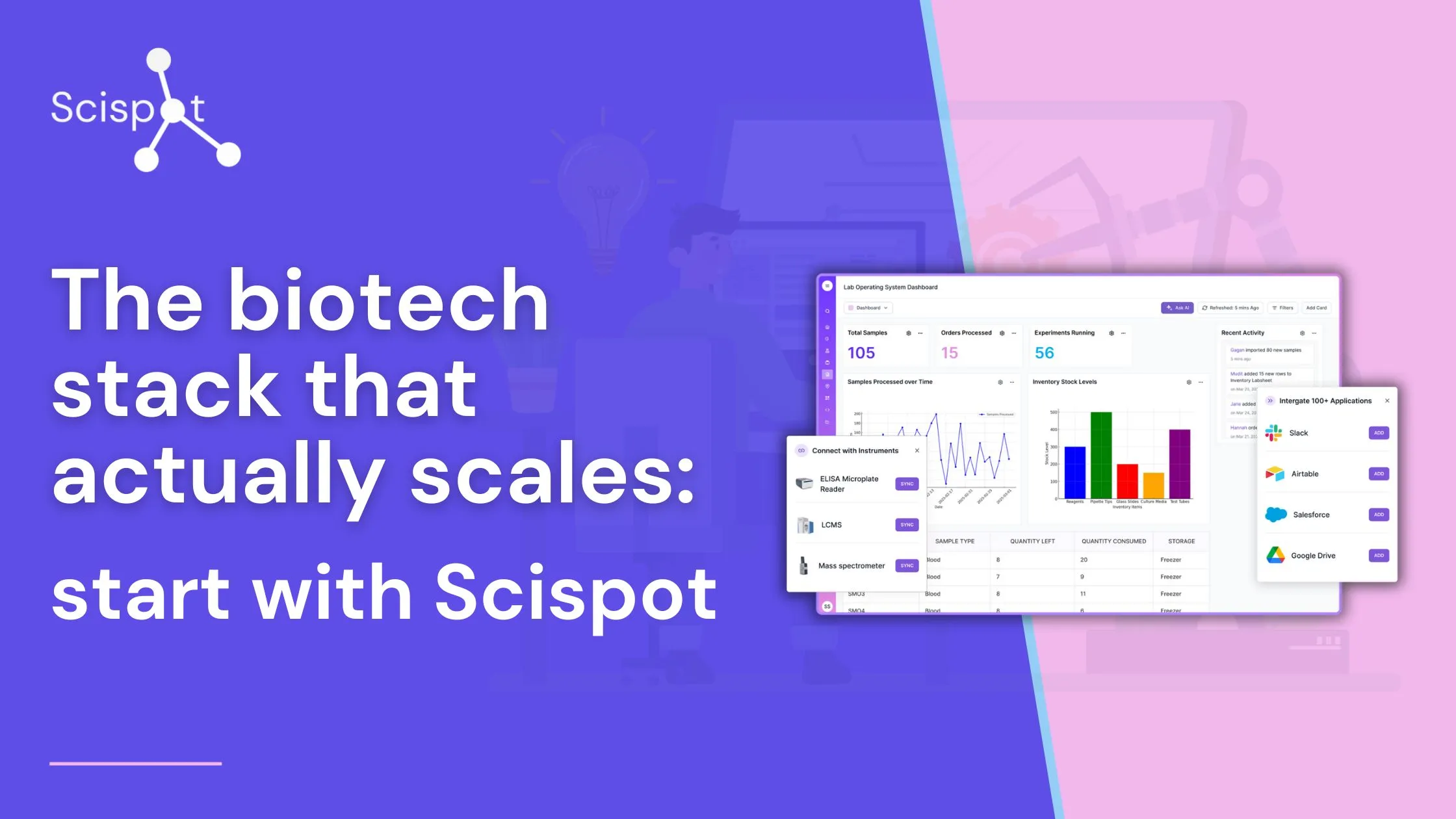
How Scispot Strengthens Biotech Quality Assurance
Scispot provides a modern, AI-powered platform that streamlines QA biotech and QC biotech processes, offering advantages over traditional QA/QC systems in several key ways.
Integrated compliance management vs. Manual compliance tracking
Traditional QA/QC systems often rely on paper-based records or disconnected spreadsheets, increasing the risk of human error and regulatory non-compliance. alt-LIMS automates compliance tracking with built-in audit trails, electronic signatures, and real-time validation protocols. This ensures adherence to GxP, FDA 21 CFR Part 11, and ISO standards, reducing the risk of regulatory violations while improving audit readiness.
AI-driven quality control vs. Reactive QC processes
Many legacy systems use reactive approaches, identifying quality issues only after batch testing is complete. Scispot, through alt-ELN, leverages AI-driven QC checks that analyze large datasets in real time, flag inconsistencies, and trigger alerts before issues escalate. This allows biotech firms to improve batch consistency, minimize recalls, and enhance product safety proactively.
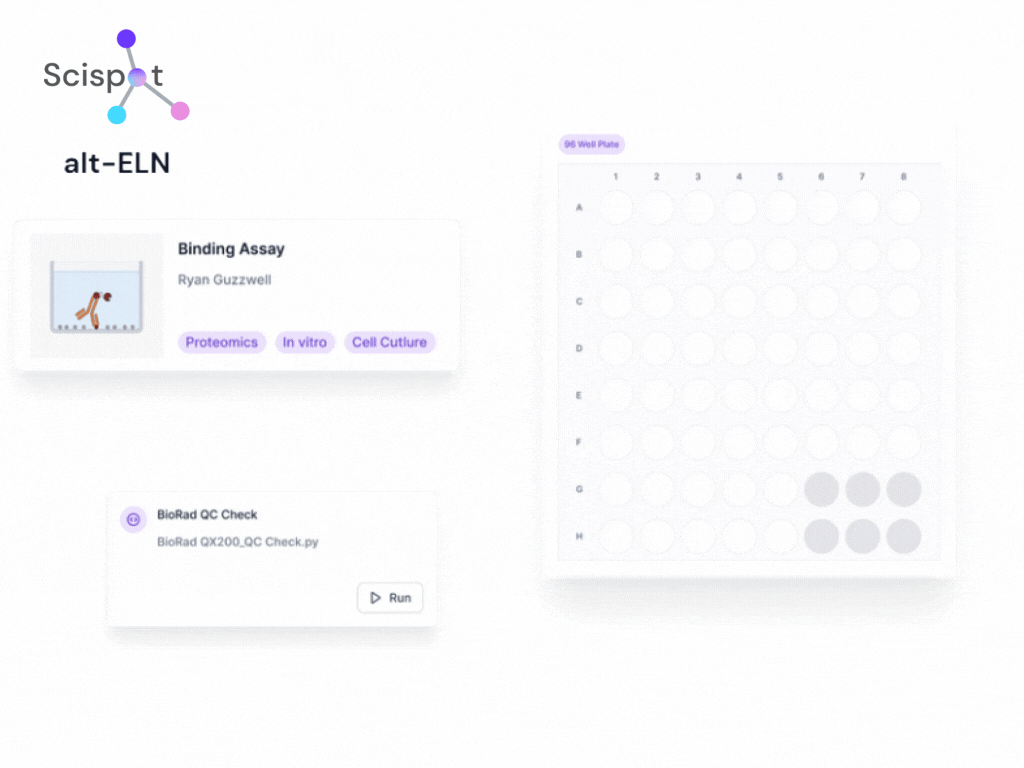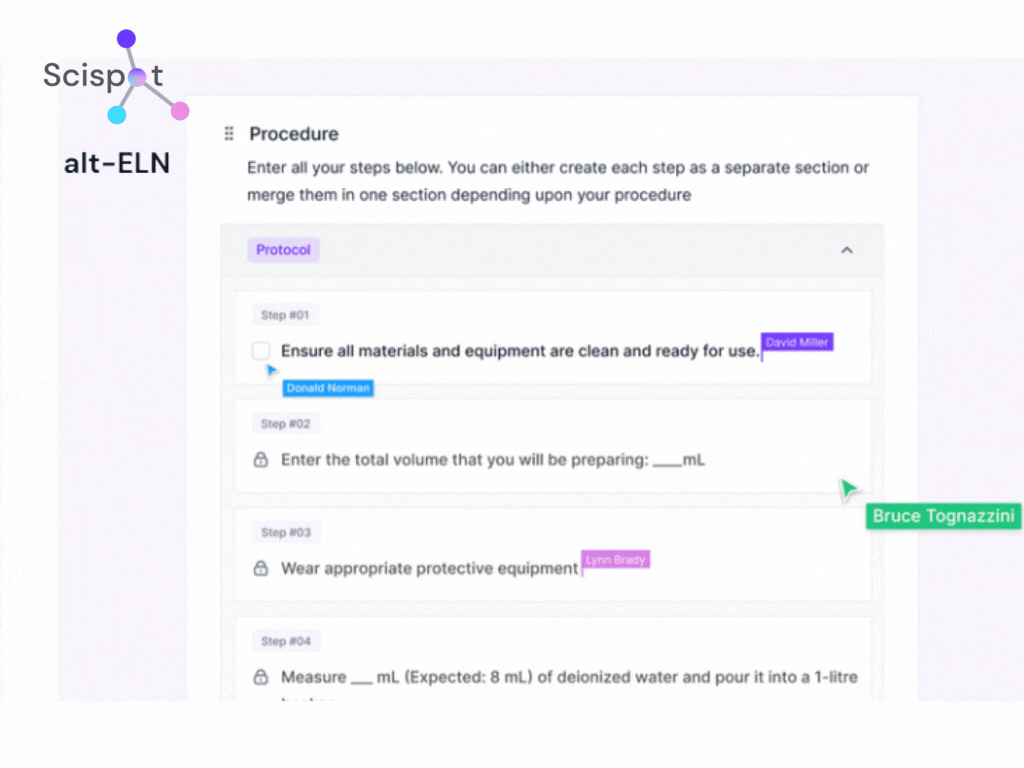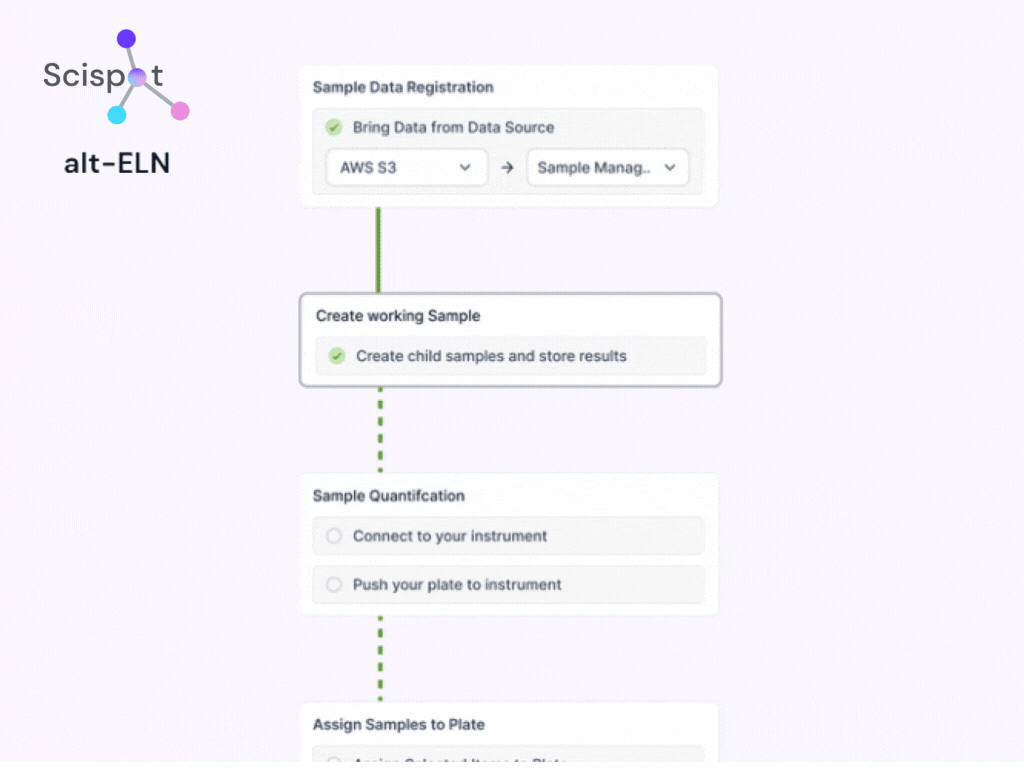
Centralized data repository vs. Siloed systems
In traditional setups, QA/QC data is often spread across multiple platforms or stored in isolated lab notebooks, making traceability and audits difficult. alt-SDMS centralizes all QA/QC records, test results, and compliance reports in a structured, searchable database. This eliminates data silos, enhances traceability, and ensures seamless collaboration across teams and locations.
Automated reporting and real-time dashboards vs. Static, manual reporting
Legacy QA/QC reporting relies heavily on manual data entry and outdated spreadsheets, leading to inefficiencies and delays. alt-LIS provides AI-powered analytics and customizable dashboards, enabling biotech firms to generate real-time insights, track process deviations, and proactively address quality risks before they impact production.
Seamless integration with lab systems vs. Limited interoperability
Traditional LIMS and ELN systems often require extensive customization to integrate with external lab equipment and digital workflows. LabOS offers API-driven interoperability with laboratory instruments, cloud storage, and third-party applications. This ensures smooth data exchange, eliminating the need for manual data entry and reducing integration costs.

By transitioning from conventional QA/QC methods to Scispot’s AI-driven, automated solution, biotech firms can improve compliance, reduce errors, and accelerate innovation, making their workflows more efficient and scalable.
Why Choose Scispot for Quality Assurance?
It ensures real-time data synchronization, enabling faster decision-making and improved regulatory readiness.
With built-in compliance automation, Scispot reduces the burden of manual documentation, lowering the risk of human errors and regulatory non-compliance.
Scispot’s AI-driven analytics optimize workflows, reduce batch failures, and enhance predictive quality control, making it a future-ready solution for growing biotech firms.
Scispot provides a fully configurable, cloud-based solution that adapts to any biotech lab’s QA/QC needs. It ensures real-time data synchronization, enabling faster decision-making and improved regulatory readiness.
Its scalable infrastructure allows both startups and enterprise labs to expand without worrying about data silos or system incompatibility.
Book a Demo
Want to see how Scispot can transform your quality assurance biotech workflows? Book a demo today and experience the future of biotech QA/QC automation.
.gif)