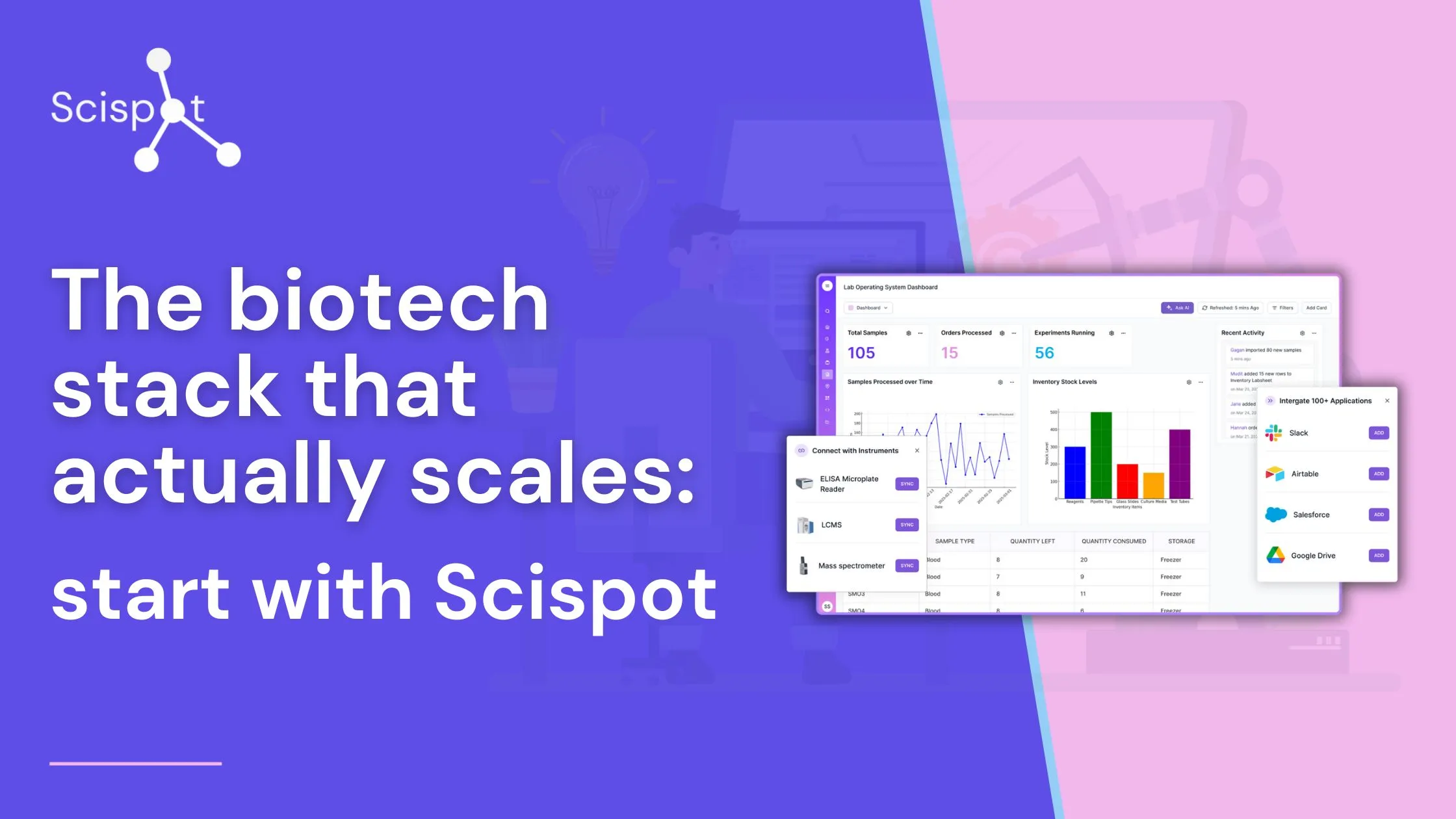
Achieving ISO 15189 accreditation is a significant milestone for any laboratory, signifying a commitment to quality and competence in medical laboratory services. The path to this accreditation involves implementing a robust Laboratory Information System (LIS) that aligns with ISO 15189 standards. Scispot offers a comprehensive LIS solution designed to support your lab's journey to accreditation, with features tailored to meet the specific needs of modern laboratories.
The Importance of ISO 15189
ISO 15189:2022 specifies the quality management system requirements particular to medical laboratories. It is crucial for ensuring the reliability and accuracy of test results, which is foundational to patient care. Laboratories pursuing this certification must demonstrate competence in their operations and adherence to stringent quality assurance protocols.
Key Phases and Steps for ISO 15189 Preparation
Phase 1: Initial Assessment and Planning
- Gap Analysis
- Conduct a thorough gap analysis to understand where your lab currently stands in relation to ISO 15189 requirements.
- Identify areas that need improvement.
- Project Planning
- Develop a detailed project plan outlining the steps, resources, and timeline required to achieve ISO 15189 accreditation.
Phase 2: Implementation
- Quality Management System (QMS) Development
- Establish a QMS that complies with ISO 15189. This includes creating policies, procedures, and documentation that align with the standard's requirements.
- Implement SOPs for all laboratory processes.
- Training and Competence
- Train all staff on the new QMS, ISO 15189 requirements, and the specific roles they will play in maintaining compliance.
- Ensure that all personnel are competent to perform their assigned tasks.
- LIS Integration
- Implement platform like Scispot’s LIS to support quality assurance processes, manage patient data securely, and document SOPs effectively.
Phase 3: Internal Audit and Review
- Internal Audits
- Conduct internal audits to verify compliance with ISO 15189. Identify non-conformities and areas for improvement.
- Use audit findings to make necessary adjustments and improvements.
- Management Review
- Perform regular management reviews to assess the effectiveness of the QMS and make strategic decisions for continuous improvement.
Phase 4: External Assessment and Accreditation
- External Audit
- Engage an accredited external body to perform a formal assessment of your lab's compliance with ISO 15189.
- Address any findings or non-conformities identified during the external audit.
- Accreditation and Certification
- Once compliance is confirmed, obtain ISO 15189 accreditation. Maintain ongoing compliance through continuous improvement and regular reviews.

Main Controls for ISO 15189 Compliance
Key Clauses and Requirements
- Management Requirements (Clause 4)
- Organization and Management Responsibility (4.1): Define the structure and responsibilities within the lab.
- Quality Management System (4.2): Establish and maintain a QMS.
- Document Control (4.3): Ensure proper documentation and control of procedures and records.
- Service Agreements (4.4): Manage contracts and agreements with clients and suppliers.
- Examination by Referral Laboratories (4.5): Maintain the quality of referred tests.
- Advisory Services (4.6): Provide consultation on the selection and interpretation of tests.
- Resolution of Complaints (4.8): Implement a process for handling complaints.
- Technical Requirements (Clause 5)
- Personnel (5.1): Ensure that staff have the necessary competence and training.
- Accommodation and Environmental Conditions (5.2): Maintain suitable facilities and environmental conditions.
- Laboratory Equipment (5.3): Properly manage and calibrate equipment.
- Pre-examination Processes (5.4): Ensure the integrity of samples before testing.
- Examination Processes (5.5): Conduct tests accurately and reliably.
- Assuring Quality of Examination Results (5.6): Implement quality control measures.
- Post-examination Processes (5.7): Handle results and reports appropriately.
Reporting Needs for Certification
- Quality Management Reports
- Document the implementation and effectiveness of the QMS.
- Include audit reports, management reviews, and corrective action records.
- Performance Metrics
- Report on key performance indicators (KPIs) such as turnaround times, error rates, and customer satisfaction.
- Compliance Documentation
- Maintain records of all procedures, SOPs, training records, and audit findings.
- Corrective and Preventive Actions (CAPA)
- Document CAPA activities to address non-conformities and improve processes.

How Scispot LIS Supports the Process
1. Comprehensive Quality Management System (QMS)
- Integrated QMS: Scispot LIS incorporates a comprehensive QMS that helps laboratories establish, maintain, and continually improve their quality management practices, which is a core requirement of ISO 15189 (Clause 4.2).
2. Documentation and Control
- Document Management: The LIS provides robust document control features, ensuring that all SOPs, policies, and procedures are properly documented, version-controlled, and easily accessible. This aligns with the document control requirements of ISO 15189 (Clause 4.3).
3. Quality Assurance and Continuous Improvement
- Automated Quality Control: Scispot LIS includes tools for automated quality control, allowing labs to monitor and record quality indicators continuously. This supports the technical requirements for quality assurance in ISO 15189 (Clause 5.6).
4. Training and Competence Management
- Training Modules: The LIS offers integrated training modules and competency assessment tools, ensuring that all staff members are adequately trained and their competencies are regularly evaluated. This addresses the personnel requirements of ISO 15189 (Clause 5.1).
5. Secure Data Management
- Data Security: Scispot ensures that all patient data is handled securely, with encryption and access controls in place to protect data integrity and confidentiality. This meets the data security requirements outlined in ISO 15189.
6. Comprehensive Laboratory Management
- Workflow Automation: The LIS automates and streamlines the entire laboratory workflow, from sample collection to result reporting. This ensures that all processes are efficient, standardized, and compliant with ISO 15189 standards (Clauses 5.4 to 5.7).
7. Reporting and Audit Trails
- Detailed Reporting: Scispot LIS generates detailed reports on lab performance, compliance, and quality management activities. It maintains audit trails for all actions and changes, providing transparency and traceability required for ISO 15189 certification.

Additional Features Tailored for Modern Labs
Cloud-Based Solution for Global Accessibility and Control
- Global Accessibility: Scispot’s cloud-based platform ensures that your lab data is accessible from anywhere in the world, providing real-time control and oversight.
Scalability
- Growth Support: The system is designed to scale with your business, accommodating increasing data volumes and expanding laboratory operations without compromising performance.
User-Friendly Interface
- Ease of Use: With an interface as intuitive as Salesforce or Workday, Scispot LIS reduces the learning curve for lab personnel, enabling them to focus on their critical tasks.
Secure Handling of Patient Data
- Data Protection: Scispot utilizes EU-based servers and robust encryption methods to ensure the highest levels of data security and compliance with GDPR.
Integration Capabilities
- Seamless Integration: The LIS can integrate with various lab equipment, including qPCR machines, facilitating a unified and efficient workflow.
Workflow Automation
- End-to-End Automation: Automate your workflow from order entry to report generation, enhancing efficiency and reducing manual errors.
Customization Options
- Tailored Solutions: Scispot offers customization options to fit specific processes, such as two-step reporting, ensuring that the system meets your unique operational needs.
AI Integration Potential
- Future-Proofing: The platform is designed with future AI integration in mind, enabling automated report generation and advanced data analysis capabilities.
Flexibility for Global Collaboration
- Partner Lab Collaboration: Scispot’s flexibility allows for seamless collaboration with partner labs globally, ensuring consistent quality and compliance across all locations.
Patient/Doctor Portals
- Enhanced Communication: Options for patient and doctor portals or integration with existing systems improve communication and data sharing, enhancing overall service quality.
Regional Differences in ISO Certification
While ISO 15189 standards are globally recognized, there may be regional variations in how these standards are implemented and audited:
United States
- Accreditation Bodies: In the US, accreditation bodies such as the College of American Pathologists (CAP) and the American Association for Laboratory Accreditation (A2LA) often conduct assessments.
- Compliance: US laboratories may need to align ISO 15189 requirements with other standards like CLIA (Clinical Laboratory Improvement Amendments).
Europe
- Accreditation Bodies: European laboratories typically work with national accreditation bodies that are part of the European co-operation for Accreditation (EA), such as UKAS in the UK.
- Data Protection: European labs must ensure compliance with GDPR for data protection, which is a significant aspect when handling patient data.

Conclusion
Achieving ISO 15189 accreditation is within reach with the right tools and systems in place. Scispot’s LIS provides a solid foundation for meeting ISO 15189 requirements, ensuring that your lab operates at the highest standards of quality and competence.
By choosing Scispot, you are not only investing in a top-tier LIS but also partnering with a team dedicated to your success. Let us support you on your journey to ISO 15189 accreditation, ensuring that your lab delivers the highest quality results with confidence and precision.
For more detailed information on the ISO 15189 standards and the benefits of implementing Scispot’s LIS, you can refer to resources like the CDC's Laboratory Quality Management System Handbook and other related publications.




.png)